Racial differences in bone mineral density and fractures in men receiving androgen deprivation therapy for prostate cancer.
Alicia K Morgans, Michael L Hancock, K Gary Barnette, Mitchell S Steiner, Ronald A Morton, Matthew R Smith
Index: J. Urol. 187(3) , 889-93, (2012)
Full Text: HTML
Abstract
Whether race influences bone loss and fracture risk during androgen deprivation therapy for prostate cancer is unknown. Using data from a prospective clinical trial we compared bone mineral density and fracture between African-American and Caucasian men receiving androgen deprivation therapy.A total of 516 subjects were in the placebo group of a 2-year randomized placebo controlled fracture prevention trial, and were African-American (68) or Caucasian (448). We compared baseline characteristics, changes in bone mineral density and rates of new fractures between races.Compared to Caucasian men, African-American men had higher baseline hip bone mineral density (mean ± SD 0.98 ± 0.15 vs 0.91 ± 0.15 gm/m(2), p = 0.001) and similar spine bone mineral density (1.09 ± 0.22 vs 1.11 ± 0.22, p = 0.51). There was no difference in prevalent vertebral fractures between African-American and Caucasian men (7.4% vs 15.0%, p = 0.13). The percentage change in hip bone mineral density at 2 years was similar between African-American and Caucasian men (mean ± SE -2.21% ± 0.59% vs -2.54% ± 0.26%, p = 0.65). Changes in bone mineral density of the lumbar spine were also similar between African-American and Caucasian men (-1.74% ± 0.69% vs -1.30% ± 0.33%, p = 0.64). No new vertebral fractures were reported in African-American men but 2 fractures were reported in Caucasian men.In a clinical trial African-American men receiving androgen deprivation therapy for prostate cancer have a greater hip bone mineral density and tended to have fewer prevalent vertebral fractures than Caucasian men. Despite a lower baseline risk of osteoporosis and fracture, African-American men experience a decrease in bone mineral density similar to that of Caucasian men.Copyright © 2012 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
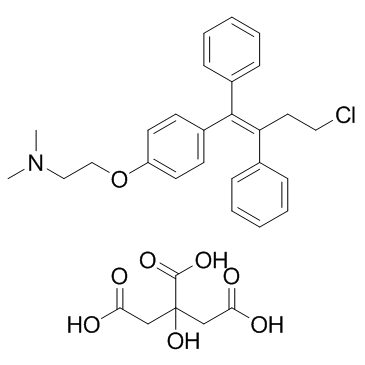 |
Toremifene Citrate
CAS:89778-27-8 |
C32H36ClNO8 |
|
Use of SERMs for treatment in postmenopausal women
2014-07-01 [J. Steroid Biochem. Mol. Biol. 142 , 142-54, (2014)] |
|
Novel off-target effect of tamoxifen--inhibition of acid cer...
2013-12-01 [Biochim. Biophys. Acta 1831(12) , 1657-64, (2013)] |
|
Prostate cancer diagnosis among men with isolated high-grade...
2013-02-10 [J. Clin. Oncol. 31(5) , 523-9, (2013)] |
|
Toremifene to reduce fracture risk in men receiving androgen...
2013-01-01 [J. Urol. 189(1 Suppl) , S45-50, (2013)] |
|
Toremifene decreases vertebral fractures in men younger than...
2011-12-01 [J. Urol. 186(6) , 2239-44, (2011)] |