Clinical efficacy of sustained-release buprenorphine with meloxicam for postoperative analgesia in beagle dogs undergoing ovariohysterectomy.
Elizabeth A Nunamaker, DeAnne F Stolarik, Junli Ma, Amanda S Wilsey, Gary J Jenkins, Chris L Medina
Index: J. Am. Assoc. Lab. Anim. Sci. 53(5) , 494-501, (2014)
Full Text: HTML
Abstract
The goal of the current study was to compare the efficacy, adverse effects, and plasma buprenorphine concentrations of sustained-release buprenorphine (SRB) and buprenorphine after subcutaneous administration in dogs undergoing ovariohysterectomy. In a prospective, randomized, blinded design, 20 healthy adult female Beagle dogs underwent routine ovariohysterectomy and received multimodal analgesia consisting of meloxicam and one of two buprenorphine formulations. Dogs were randomly assigned to receive either SRB (0.2 mg/kg SC, once) or buprenorphine (0.02 mg/kg SC every 12 h for 3 d). Blinded observers assessed all dogs by using sedation scores, pain scores, temperature, HR, RR, and general wellbeing. Dogs were provided rescue analgesia with 0.02 mg/kg buprenorphine SC if the postoperative pain score exceeded a prede- termined threshold. Blood samples were collected, and mass spectrometry was used to determine plasma buprenorphine concentrations. Data were analyzed with a linear mixed model and Tukey-Kramer multiple comparison. Age, body weight, anesthetic duration, surgical duration, sevoflurane concentration, and cardiorespiratory variables did not differ significantly between groups. Dogs in both formulation groups had comparable postoperative sedation and pain scores. One dog from each formulation group had breakthrough pain requiring rescue analgesia. Plasma buprenorphine concentrations remained above a hypothesized therapeutic concentration of 0.6 ng/mL for 136.0 ± 11.3 and 10.67 ± 0.84 h for SRB and buprenorphine, respectively. Based on the results of this study, multimodal analgesic regimens consisting of meloxicam and either buprenorphine or SRB are equally efficacious in managing pain associated with an ovariohysterectomy and show comparable side effects.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
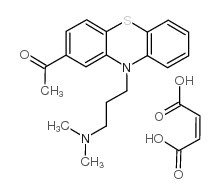 |
Acepromazine maleate
CAS:3598-37-6 |
C23H26N2O5S |
|
The in vitro and in vivo profile of aclidinium bromide in co...
2014-08-01 [Pulm. Pharmacol. Ther. 28(2) , 114-21, (2014)] |
|
Determination of 15 sedative residues in mutton by rapid res...
2015-02-01 [J. Sci. Food Agric. 95(3) , 598-606, (2015)] |
|
Development and in-vivo evaluation of ondansetron gels for t...
2015-06-01 [Drug Dev. Ind. Pharm. 41 , 1030-6, (2015)] |
|
Effect of doxycycline on contralateral canine cranial crucia...
2015-01-01 [Vet. Comp. Orthop. Traumatol. 28 , 371-8, (2015)] |
|
Experimental dog model for assessment of fasting and postpra...
2015-07-01 [Lab. Anim. 49 , 228-40, (2015)] |