Syntheses of 5,6,7- and 5,7,8-trioxygenated 3',4'-dihydroxyflavones having alkoxy groups and their inhibitory activities against arachidonate 5-lipoxygenase.
T Horie, M Tsukayama, H Kourai, C Yokoyama, M Furukawa, T Yoshimoto, S Yamamoto, S Watanabe-Kohno, K Ohata
Index: J. Med. Chem. 29(11) , 2256-62, (1986)
Full Text: HTML
Abstract
Arachidonate 5-lipoxygenase plays a pivotal role in the biosynthesis of leukotrienes. Cirsiliol (3',4',5-trihydroxy-6,7-dimethoxyflavone), a selective inhibitor of the enzyme, was derivatized by introducing alkyl groups of various chain lengths at positions 5, 6, 7, and 8 of the A ring of the flavone skeleton. Modification of the positions 5 and 6 with an alkyl group of 5-10 carbons markedly decreased the IC50 values for 5-lipoxygenase inhibition to the order of 10 nM. As tested with 5- or 6-hexyloxy derivatives, a relatively selective inhibition of 5-lipoxygenase was shown. Inhibition of 12-lipoxygenase required much higher concentrations of these compounds, and cyclooxygenase was not inhibited. Modification of positions 7 and 8 did not increase the inhibitory effect of most flavone compounds.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
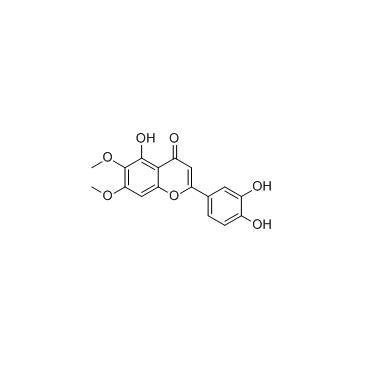 |
Cirsiliol
CAS:34334-69-5 |
C17H14O7 |
|
[Chemical constituents of Eupatorium lindleyanum].
2012-04-01 [Zhongguo Zhong Yao Za Zhi 37(7) , 937-40, (2012)] |
|
[Determination of flavonoids in buds of Herba Artemisiae Sco...
2005-04-01 [Zhongguo Zhong Yao Za Zhi 30(8) , 591-4, (2005)] |
|
Potent and selective 5-lipoxygenase inhibitors: cirsiliol an...
1985-01-01 [Adv. Prostaglandin. Thromboxane. Leukot. Res. 15 , 217-9, (1985)] |
|
[Studies on chemical constituents in buds of Artemisia scopa...
2002-03-01 [Zhongguo Zhong Yao Za Zhi 27(3) , 202-4, (2002)] |
|
GABA(A)-receptor ligands of flavonoid structure.
2002-08-01 [Curr. Top. Med. Chem 2(8) , 853-67, (2002)] |