Arachidonate 5-lipoxygenase and its new inhibitors.
S Yamamoto, T Yoshimoto, M Furukawa, T Horie, S Watanabe-Kohno
Index: J. Allergy Clin. Immunol. 74(3 Pt 2) , 349-52, (1984)
Full Text: HTML
Abstract
The 5-lipoxygenases of guinea pig peritoneal polymorphonuclear leukocytes and of rat basophilic leukemia cells have been solubilized, purified partially by affinity chromatography, and shown to convert arachidonic acid principally to 5-hydroperoxy-6,8,11,14- eicosatetraenoic acid. The activity of both 5-lipoxygenases is calcium dependent and enhanced by adenosine triphosphate and other nucleotides in the presence of calcium ion. Both 5-lipoxygenase activity and leukotriene generation by sensitized guinea pig lung tissue challenged with antigen were suppressed substantially by specific benzoquinone and flavonoid inhibitors. The in vivo significance of the findings will be explored with potent and selective lipoxygenase inhibitors, which are delineated in the 5-lipoxygenase model systems.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
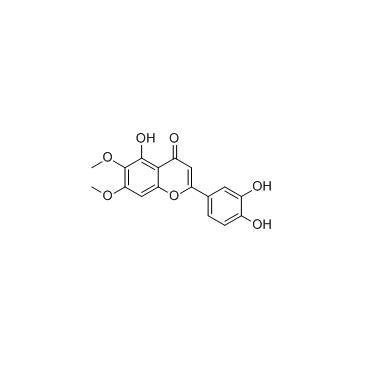 |
Cirsiliol
CAS:34334-69-5 |
C17H14O7 |
|
[Chemical constituents of Eupatorium lindleyanum].
2012-04-01 [Zhongguo Zhong Yao Za Zhi 37(7) , 937-40, (2012)] |
|
[Determination of flavonoids in buds of Herba Artemisiae Sco...
2005-04-01 [Zhongguo Zhong Yao Za Zhi 30(8) , 591-4, (2005)] |
|
Potent and selective 5-lipoxygenase inhibitors: cirsiliol an...
1985-01-01 [Adv. Prostaglandin. Thromboxane. Leukot. Res. 15 , 217-9, (1985)] |
|
[Studies on chemical constituents in buds of Artemisia scopa...
2002-03-01 [Zhongguo Zhong Yao Za Zhi 27(3) , 202-4, (2002)] |
|
GABA(A)-receptor ligands of flavonoid structure.
2002-08-01 [Curr. Top. Med. Chem 2(8) , 853-67, (2002)] |