Reaction of lipids containing hydroxy groups with trimethylsulfonium hydroxide: formation of O-methyl derivatives.
K Vosmann, E Schulte, E Klein, N Weber
Index: Lipids 31(3) , 349-52, (1996)
Full Text: HTML
Abstract
Base-catalyzed transesterification of acyl lipids with trimethylsulfonium hydroxide (TMSH) is an easy and convenient method for the preparation of fatty acid methyl esters (FAME) for gas chromatography (GC) analyses. We have found, however, that lipids containing hydroxy groups are partially converted to the corresponding O-methyl ether derivatives which may interfere with FAME in GC separations. For example, long-chain alcohols are found to be converted to alkyl methyl ethers, rac-1-O-alkylglycerols to the corresponding 2-O- and 3-O-monomethyl ethers, as well as 2,3-di-O-methyl ethers, hydroxy fatty acids to methoxy FAME, and cholesterol to cholesteryl 3 beta-methyl ether. From our results, it is obvious that TMSH derivatization method is not recommended without limitation for lipids containing hydroxy groups; it may be, however, of some diagnostic value for the analysis of such lipids by GC/mass spectrometry.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
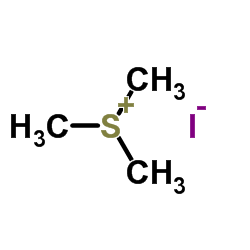 |
Trimethylsulfonium iodide
CAS:2181-42-2 |
C3H9IS |
|
Interferences in the direct quantification of bisphenol S in...
2013-02-01 [J. Chromatogr. A. 1275 , 70-7, (2013)] |
|
A high-throughput fatty acid profiling screen reveals novel ...
2014-11-01 [Eukaryotic Cell 13(11) , 1431-8, (2014)] |
|
S-methyl derivatives from thiol compounds by the pyrolytic r...
1998-10-01 [Lipids 33(10) , 1037-41, (1998)] |
|
Evaluation of the cholinomimetic actions of trimethylsulfoni...
2002-04-01 [Braz. J. Med. Biol. Res. 35(4) , 485-91, (2002)] |
|
Prebiotic methylation and the evolution of methyl transfer r...
2000-12-01 [Orig. Life Evol. Biosph. 30(6) , 539-48, (2000)] |