Rearrangement of 5S, 12S-dihydroxy-6,8,10,14-(E,Z,E,Z)-eicosatetraenoic acid during gas chromatography: formation of a cyclohexadiene derivative.
P Borgeat, S Pilote
Index: Prostaglandins 35(5) , 723-31, (1988)
Full Text: HTML
Abstract
The 5S, 12S-dihydroxy-6,8,10,14-(E,Z,E,Z,)-eicosatetraenoic acid, a product of double dioxygenation of arachidonic acid by lipoxygenases, undergoes severe decomposition during gas chromatography-mass spectrometric (GC-MS) analysis of the trimethylsilyl ether methyl ester derivative. The decomposition product was studied by GC-MS and identified as a cyclohexadiene derivative of the parent compound formed by ring closure at C6 and C11. Under identical GC conditions, two stereoisomers, i.e. 5S,12R-dihydroxy-6,8,10,14-(Z,E,E,Z)-eicosatetraenoic acid (leukotriene B4), and 6-trans-leukotriene B4 showed excellent chromatographic properties. These data indicated that the 5,12-dihydroxy derivative of arachidonic acid carrying the trans-cis-trans triene unit selectively undergoes cyclization during GC. These studies also provided an explanation to the controversial GC-MS data reported for this lipoxygenase product.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
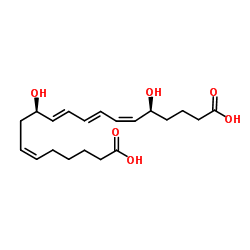 |
20-hydroxy-20-oxoleukotriene B4
CAS:80434-82-8 |
C20H30O6 |
|
The human serum metabolome.
2011-01-01 [PLoS ONE 6(2) , e16957, (2011)] |
|
Identification and biological activity of novel omega-oxidiz...
1981-07-20 [FEBS Lett. 130(1) , 107-12, (1981)] |
|
Defective metabolism of leukotriene B4 in the Sjögren-Larsso...
2001-01-15 [J. Neurol. Sci. 183(1) , 61-7, (2001)] |
|
Biosynthesis and metabolism of leukotrienes.
2007-08-01 [Biochem. J. 405(3) , 379-95, (2007)] |
|
Metabolism of leukotriene B4 by polymorphonuclear granulocyt...
1987-05-01 [Prostaglandins. Leukot. Med. 27(2-3) , 209-25, (1987)] |