Chemical Communications
2012-12-28
Total syntheses of mitragynine, paynantheine and speciogynine via an enantioselective thiourea-catalysed Pictet-Spengler reaction.
Isabel P Kerschgens, Elise Claveau, Martin J Wanner, Steen Ingemann, Jan H van Maarseveen, Henk Hiemstra
Index: Chem. Commun. (Camb.) 48(100) , 12243-5, (2012)
Full Text: HTML
Abstract
The pharmacologically interesting indole alkaloids (-)-mitragynine, (+)-paynantheine and (+)-speciogynine were synthesised in nine steps from 4-methoxytryptamine by a route featuring (i) an enantioselective thiourea-catalysed Pictet-Spengler reaction, providing the tetrahydro-β-carboline ring and (ii) a Pd-catalysed Tsuji-Trost allylic alkylation, closing the D-ring.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
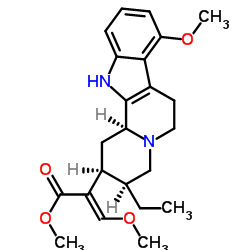 |
Mitragynine
CAS:4098-40-2 |
C29H33N5O11 |
Related Articles:
More...
|
A drug fatality involving Kratom.
2013-01-01 [J. Forensic Sci. 58 Suppl 1 , S278-9, (2013)] |
|
Orally active opioid compounds from a non-poppy source.
2013-06-27 [J. Med. Chem. 56(12) , 4840-8, (2013)] |
|
Determination of mitragynine in plasma with solid-phase extr...
2010-07-01 [Anal. Bioanal. Chem 397(5) , 2023-30, (2010)] |
|
A simple HPLC-DAD method for the detection and quantificatio...
2013-03-10 [Forensic Sci. Int. 226(1-3) , 183-7, (2013)] |
|
Analysis of mitragynine and metabolites in human urine for d...
2012-01-01 [J. Anal. Toxicol. 36(9) , 616-25, (2012)] |