| Structure | Name/CAS No. | Articles |
|---|---|---|
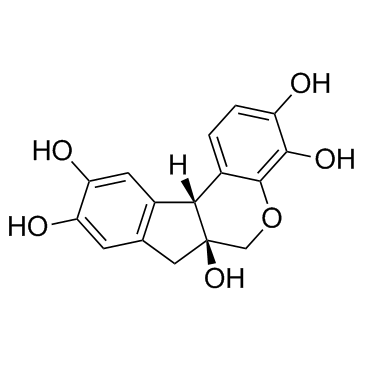 |
Hematoxylin
CAS:517-28-2 |
|
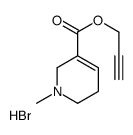 |
ARECAIDINE PROPARGYL ESTER HYDROBROMIDE (APE)
CAS:116511-28-5 |