| Structure | Name/CAS No. | Articles |
|---|---|---|
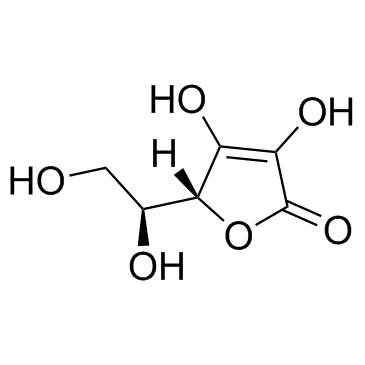 |
Ascorbic acid
CAS:50-81-7 |
|
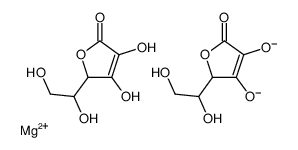 |
(+)-Magnesium L-ascorbate
CAS:15431-40-0 |
|
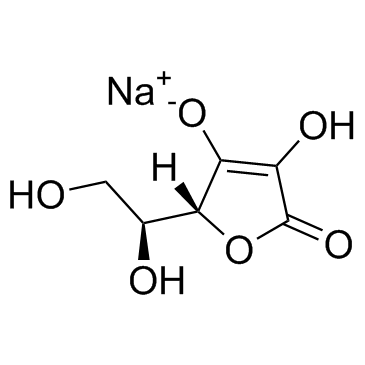 |
sodium ascorbate
CAS:134-03-2 |