Photocatalytical removal of inorganic and organic arsenic species from aqueous solution using zinc oxide semiconductor.
Nidia Rivera-Reyna, Laura Hinojosa-Reyes, Jorge Luis Guzmán-Mar, Yong Cai, Kevin O'Shea, Aracely Hernández-Ramírez
Index: Photochem. Photobiol. Sci. 12(4) , 653-9, (2013)
Full Text: HTML
Abstract
The photocatalytic removal of arsenite [As(III)] and monomethylarsonic acid [MMA(V)] was investigated in the presence of UV light (350 nm) and aqueous suspensions of ZnO synthesized by the sol-gel technique. Photocatalytic removal of these potent arsenic compounds results in the effective and rapid mineralization to less toxic inorganic arsenate [As(V)]. The effect of ZnO loading and solution pH on the treatment efficiency of the UV/ZnO photocatalytic process was evaluated. The optimal conditions for the removal of 5 mg L(-1) [As(III)] and [MMA(V)] aqueous solutions were observed at catalyst loadings of 0.25 and 0.50 g L(-1) with solution pH values of 7 and 8, respectively. Under these conditions, the activity of photocatalyst sol-gel ZnO was compared with TiO2 Degussa P25 and commercial ZnO catalyst. The results demonstrate that the high adsorption capacity of ZnO synthesized by sol-gel gives enhanced removal of arsenic species from water samples, indicating that this catalyst is a promising material for treatment of arsenic contaminated groundwater.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
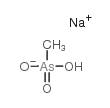 |
Sodium methanearsonate
CAS:2163-80-6 |
CH4AsNaO3 |
|
Non-chromatographic screening procedure for arsenic speciati...
2011-08-05 [Anal. Chim. Acta 699(1) , 11-7, (2011)] |
|
Urinary arsenic speciation profiles in mice subchronically e...
2011-09-01 [Kaohsiung J. Med. Sci. 27(9) , 417-23, (2011)] |
|
Arsenic speciation in saliva of acute promyelocytic leukemia...
2013-02-01 [Anal. Bioanal. Chem 405(6) , 1903-11, (2013)] |
|
Inadvertent poisoning of seven teenagers with monosodium met...
2011-03-01 [Clin. Toxicol. (Phila.) 49(3) , 167-70, (2011)] |
|
Metabolites of arsenic and increased DNA damage of p53 gene ...
2011-07-01 [Toxicol. Appl. Pharmacol. 254(1) , 41-7, (2011)] |