| Structure | Name/CAS No. | Articles |
|---|---|---|
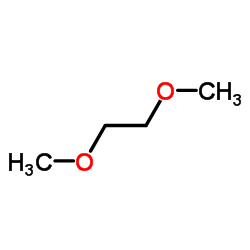 |
1,2-dimethoxyethane
CAS:110-71-4 |
|
 |
(R)-Mandelonitrile lyase
CAS:9024-43-5 |