Joint QSAR analysis using the Free-Wilson approach and quantum chemical parameters.
D B Wei, A Q Zhang, S K Han, L S Wang
Index: SAR QSAR Environ. Res. 12(5) , 471-9, (2001)
Full Text: HTML
Abstract
A new quantitative structure-activity relationship (QSAR) technique combining the Free-Wilson method and constructed quantum chemical parameters was used to simulate the aqueous solubility (Sw), 1-octanol/water partition coefficient (Kow) of 14 new synthesized benzanilide derivatives and their 96 h acute toxicity (EC50) to Daphnia magna. The mode of action of the 14 selected compounds to Daphnia magna was shown to be a complex process involving a physical partition stage and a bio-chemical reaction stage. The results also indicated that the joint (QSAR) analysis was much effective than the original Free-Wilson method and Hansch method not only in predicting properties/toxicity, but also in investigating the mode of action of chemicals.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
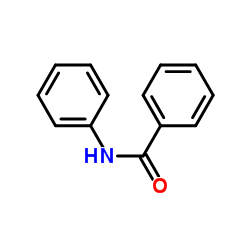 |
Benzanilide
CAS:93-98-1 |
C13H11NO |
|
Benzanilides with spasmolytic activity: chemistry, pharmacol...
2008-06-01 [Bioorg. Med. Chem. 16 , 5974-81, (2008)] |
|
Unusual spectral shifts on fast violet-B and benzanilide: Ef...
2009-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 74(2) , 484-97, (2009)] |
|
On the relationship between the substitution pattern of thio...
2002-09-01 [Il Farmaco 57(9) , 777-82, (2002)] |
|
Dual fluorescence of diphenyl carbazide and benzanilide: eff...
2005-12-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 62(4-5) , 991-9, (2005)] |
|
New amido derivatives as potential BKCa potassium channel ac...
2008-04-01 [Eur. J. Med. Chem. 43(4) , 792-9, (2008)] |