Direct binding of RalA to PKCη and its crucial role in morphological change during keratinocyte differentiation.
Yasuhito Shirai, Shoko Morioka, Megumi Sakuma, Ken-Ichi Yoshino, Chihiro Otsuji, Norio Sakai, Kaori Kashiwagi, Kazuhiro Chida, Ryutaro Shirakawa, Hisanori Horiuchi, Chikako Nishigori, Takehiko Ueyama, Naoaki Saito
Index: Mol. Biol. Cell 22(8) , 1340-52, (2011)
Full Text: HTML
Abstract
During differentiation, keratinocytes undergo a dramatic shape change from small and round to large and flat, in addition to production of proteins necessary for the formation of epidermis. It has been shown that protein kinase C (PKC) η is crucial for keratinocyte differentiation. However, its role in this process has yet to be fully elucidated. Here, we show that catalytic activity is not necessary for enlarged and flattened morphology of human keratinocytes induced by overexpression of PKCη, although it is important for gene expression of the marker proteins. In addition, we identify the small G protein RalA as a binding partner of PKCη, which binds to the C1 domain, an indispensable region for the morphological change. The binding led activation of RalA and actin depolymerization associated with keratinocyte differentiation. siRNA techniques proved that RalA is involved in not only the keratinocyte differentiation induced by PKCη overexpression but also normal keratinocyte differentiation induced by calcium and cholesterol sulfate. These results provide a new insight into the molecular mechanism of cytoskeletal regulation leading to drastic change of cell shape.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
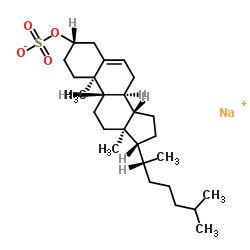 |
Cholesterol 3-Sulfate Sodium Salt
CAS:2864-50-8 |
C27H45NaO4S |
|
Investigation of stratum corneum lipid model membranes with ...
2010-07-01 [Eur. Biophys. J. 39(8) , 1167-76, (2010)] |
|
Integral hair lipid in human hair follicle.
2011-12-01 [J. Dermatol. Sci. 64(3) , 153-8, (2011)] |
|
Metabolic alterations of DHEA and cholesterol sulphates in t...
2010-07-01 [Exp. Dermatol. 19(7) , 694-6, (2010)] |
|
New applications of phospholipid vesicle-based permeation as...
2013-05-01 [J. Pharm. Sci. 102(5) , 1588-600, (2013)] |
|
Injectable nimodipine-loaded nanoliposomes: Preparation, lyo...
2011-01-01 [Int. J. Pharm. 410(1-2) , 180-7, (2011)] |