Synthesis of 6-substituted 1-phenylbenzazepines and their dopamine D(1) receptor activities.
Jing Zhang, Xuetao Chen, Leiping Yu, Xuechu Zhen, Ao Zhang
Index: Bioorg. Med. Chem. 16(21) , 9425-31, (2008)
Full Text: HTML
Abstract
A series of racemic 6-aryl substituted 1-phenylbenzazepines 7a-e, and 17a,b were prepared. All these compounds showed binding potencies compatible to or much higher than that of the prototypic (+/-)-SKF-38393 ((+/-)-1) and (+/-)-SKF-83959 (3) for the D(1) receptor. Among analogs of (+/-)-SKF-38393, compounds 7b, 7c and 7e possess 10-, 2- and 7-fold enhancement in binding for the D(1) receptor, respectively. Lower but compatible potency to that of (+/-)-1 was observed for compounds 7a and 7d. The optimal 6-substituents (m-tolyl, and 2'-naphthyl) were applied to the skeleton of (+/-)-SKF-83959 (3). The resulting compounds 17a,b displayed high affinity at the D(1) receptor, only slightly lower than that of 3. These two compounds also showed good binding at the D(2) receptor.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
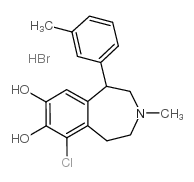 |
SKF 83959 HYDROBROMIDE
CAS:80751-85-5 |
C18H21BrClNO2 |
|
SKF83959 is a potent allosteric modulator of sigma-1 recepto...
2013-03-01 [Mol. Pharmacol. 83(3) , 577-86, (2013)] |
|
The D₁ dopamine receptor agonist, SKF83959, attenuates hydro...
2012-01-01 [Mol. Vis. 18 , 2882-95, (2012)] |
|
Phosphatidylinositol-linked novel D(1) dopamine receptor fac...
2009-08-01 [Neuropharmacology 57(2) , 164-71, (2009)] |
|
Assessment of jaw movements by magnetic sensor in relation t...
2010-04-25 [Eur. J. Pharmacol. 632(1-3) , 39-44, (2010)] |
|
Regulation of DARPP-32 phosphorylation by three distinct dop...
2008-11-01 [J. Neurochem. 107(4) , 1014-26, (2008)] |