| Structure | Name/CAS No. | Articles |
|---|---|---|
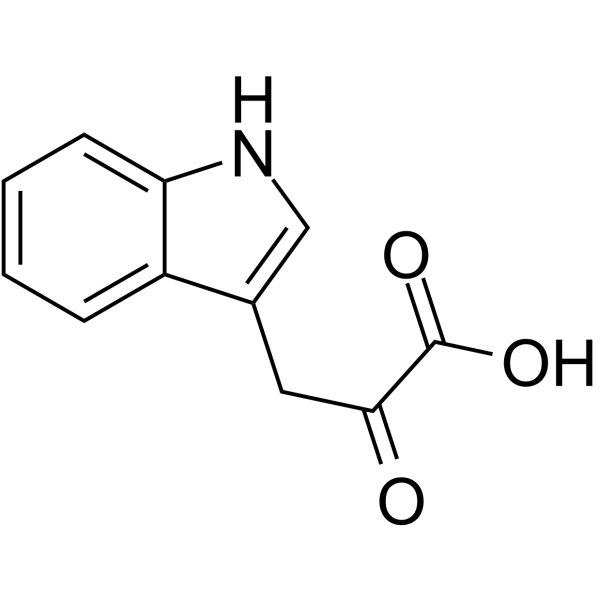 |
Indole-3-pyrubate
CAS:392-12-1 |
|
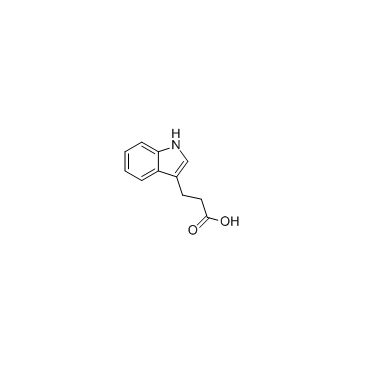 |
3-Indolepropionic acid
CAS:830-96-6 |