Liquid chromatography/mass spectrometry analysis of bifunctional electrophiles and DNA adducts from vitamin C mediated decomposition of 15-hydroperoxyeicosatetraenoic acid.
Michelle V Williams, Seon Hwa Lee, Ian A Blair
Index: Rapid Commun. Mass Spectrom. 19(6) , 849-58, (2005)
Full Text: HTML
Abstract
Reactive oxygen species convert the omega-6 polyunsaturated fatty acid arachidonic acid into 15-hydroperoxy-5,8,11,13-(Z,Z,ZE)-eicosatetraenoic acid (15-HPETE). Cyclooxygenases and lipoxygenases can also convert arachidonic acid into 15-HPETE. Vitamin C mediated decomposition of 15(S)-HPETE to protein- and DNA-reactive bifunctional electrophiles was examined by normal-phase liquid chromatography/atmospheric pressure chemical ionization/mass spectrometry (LC/APCI-MS). The individual bifunctional electrophiles, trans-4,5-epoxy-2(E)-decenal (t-EDE), cis-4,5-epoxy-2(E)-decenal (c-EDE), 4-oxo-2(E)-nonenal (ONE), and 4-hydroxy-2(E)-nonenal (HNE), exhibited protonated molecules at m/z 169, 169, 155, and 157, respectively. The MH+ ion at m/z 173 for 4-hydroperoxy-2(E)-nonenal (HPNE) was very weak with an ion corresponding to the loss of OH at m/z 156 as the major ion in the APCI mass spectrum. The bifunctional electrophiles were all separated under normal-phase LC conditions. All five bifunctional electrophiles were formed when 15-HPETE was treated with vitamin C. The LC/MS-based methodology showed that t-EDE was the major bifunctional electrophile formed during vitamin C mediated 15(S)-HPETE decomposition. Stable isotope dilution LC/MS studies revealed that this did not result in the formation of increased levels of unsubstituted etheno-dGuo adducts in calf thymus DNA when compared with 13(S)-hydroperoxy-9,10-(Z,E)-octadecadienoic acid [13(S)-HPODE], a lipid hydroperoxide derived from linoleic acid. However, the formation of heptanone-etheno-dGuo adducts in calf thymus DNA was reduced when compared with the 13(S)-HPODE. This was attributed to the reduced formation of ONE from 15-HPETE when compared with its formation from 13-HPODE. In contrast to reactions with dGuo or DNA conducted using 13(S)-HPODE, no carboxy-containing adducts were observed with 15(S)-HPETE.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
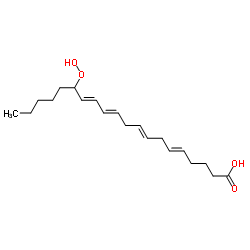 |
15-hydroperoxy-5,8,11,13-eicosatetraenoic acid
CAS:70981-96-3 |
C20H32O4 |
|
The human serum metabolome.
2011-01-01 [PLoS ONE 6(2) , e16957, (2011)] |
|
Novel role of lipoxygenases in the inflammatory response: pr...
2005-03-15 [J. Immunol. 174(6) , 3169-72, (2005)] |
|
Nature of the cardiomyocyte injury induced by lipid hydroper...
1995-11-01 [Cardiovasc. Res. 30(5) , 648-55, (1995)] |
|
On the relationships of substrate orientation, hydrogen abst...
2005-11-18 [J. Biol. Chem. 280(46) , 38756-66, (2005)] |
|
Conditional expression of 15-lipoxygenase-1 inhibits the sel...
2004-07-02 [J. Biol. Chem. 279(27) , 28028-35, (2004)] |