Switching between agonists and antagonists at CRTh2 in a series of highly potent and selective biaryl phenoxyacetic acids
Tim Luker, Roger Bonnert, Jerzy Schmidt, Carol Sargent, Stuart W. Paine, Stephen Thom, Gary Pairaudeau, Anil Patel, Rukhsana Mohammed, Elizabeth Akam, Iain Dougall, Andrew M. Davis, Phil Abbott, Steve Brough, Ian Millichip, Thomas McInally, Tim Luker, Roger Bonnert, Jerzy Schmidt, Carol Sargent, Stuart W. Paine, Stephen Thom, Gary Pairaudeau, Anil Patel, Rukhsana Mohammed, Elizabeth Akam, Iain Dougall, Andrew M. Davis, Phil Abbott, Steve Brough, Ian Millichip, Thomas McInally, Tim Luker, Roger Bonnert, Jerzy Schmidt, Carol Sargent, Stuart W. Paine, Stephen Thom, Gary Pairaudeau, Anil Patel, Rukhsana Mohammed, Elizabeth Akam, Iain Dougall, Andrew M. Davis, Phil Abbott, Steve Brough, Ian Millichip, Thomas McInally
Index: Bioorg. Med. Chem. Lett. 21(12) , 3616-21, (2011)
Full Text: HTML
Abstract
A novel series of biaryl phenoxyacetic acids was discovered as potent, selective antagonists of the chemoattractant receptor-homologous expressed on Th2 lymphocytes receptor (CRTh2 or DP2). A hit compound 4 was discovered from high throughput screening. Modulation of multiple aryl substituents afforded both agonists and antagonists, with small changes often reversing the mode of action. Understanding the complex SAR allowed design of potent antagonists such as potential candidate 34.Copyright © 2011 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
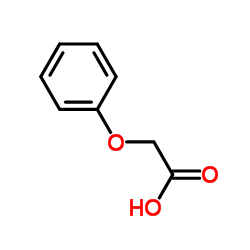 |
Phenoxyacetic acid
CAS:122-59-8 |
C8H8O3 |
|
Synthesis, characterization and antibacterial activity of az...
2007-01-01 [Molecules 12(2) , 245-54, (2007)] |
|
Penicillin V acylase from Pectobacterium atrosepticum exhibi...
2015-08-01 [Int. J. Biol. Macromol. 79 , 1-7, (2015)] |
|
Correlations between chromatographic parameters and bioactiv...
2014-08-01 [J. Chromatogr. Sci. 52 , 676-84, (2014)] |
|
Development of a physiologically-based pharmacokinetic model...
2015-11-01 [Regul Toxicol Pharmacol 73 , 530-43, (2015)] |
|
Characterization of 2,4-dichlorophenoxyacetic acid and 2,4,5...
2013-04-01 [FEMS Microbiol. Ecol. 84(1) , 124-32, (2013)] |