Retention behavior of phenoxyacetic herbicides on a molecularly imprinted polymer with phenoxyacetic acid as a dummy template molecule.
Huiting Zhang, Tao Song, Wei Zhang, Wei Hua, Canping Pan
Index: Bioorg. Med. Chem. 15(18) , 6089-95, (2007)
Full Text: HTML
Abstract
Molecular imprinted polymers (MIPs) binding with phenoxyacetic acid (PA) as a dummy template molecule were synthesized via thermal initiation in aqueous medium. The retention behaviors of benzoic acid (BA), PA, 2-methyl-4-chlorophenoxyacetic acid (MCPA), 4-chlorophenoxyacetic acid (4-CPA), and 2,4-dichlorophenoxyacetic acid (2,4-D) on this MIP column indicate that this material can selectively retain phenoxyacetic herbicides. To investigate these recognition mechanisms, the interactions between the functional monomer 4-vinylpyridine (4-VP) and PA or 2,4-D were investigated by computational modeling. (1)H NMR spectroscopy of 2,4-D titrated by 4-VP was recorded. The chemical shift of the 2,4-D acidic proton (12.15-14.32ppm) shows the existence of the ion-pair interaction. This kind of polymers could be useful as stationary phases to extract 2,4-D, 4-CPA or MCPA and avoid leakage of a trace amount of target analyte remaining in the MIPs.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
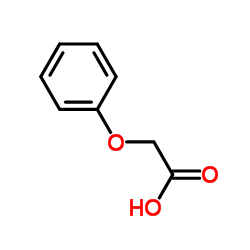 |
Phenoxyacetic acid
CAS:122-59-8 |
C8H8O3 |
|
Synthesis, characterization and antibacterial activity of az...
2007-01-01 [Molecules 12(2) , 245-54, (2007)] |
|
Penicillin V acylase from Pectobacterium atrosepticum exhibi...
2015-08-01 [Int. J. Biol. Macromol. 79 , 1-7, (2015)] |
|
Correlations between chromatographic parameters and bioactiv...
2014-08-01 [J. Chromatogr. Sci. 52 , 676-84, (2014)] |
|
Development of a physiologically-based pharmacokinetic model...
2015-11-01 [Regul Toxicol Pharmacol 73 , 530-43, (2015)] |
|
Characterization of 2,4-dichlorophenoxyacetic acid and 2,4,5...
2013-04-01 [FEMS Microbiol. Ecol. 84(1) , 124-32, (2013)] |