Bioorganic & Medicinal Chemistry Letters
2006-06-15
Substituted pyrrolidine-2,4-dicarboxylic acid amides as potent dipeptidyl peptidase IV inhibitors.
Ting-Yueh Tsai, Mohane Selvaraj Coumar, Tsu Hsu, Hsing-Pang Hsieh, Chia-Hui Chien, Chiung-Tong Chen, Chung-Nien Chang, Yu-Kang Lo, Ssu-Hui Wu, Chung-Yu Huang, Yu-Wen Huang, Min-Hsien Wang, Hsin-Yi Wu, Hong-Jen Lee, Xin Chen, Yu-Sheng Chao, Weir-Torn Jiaang
Index: Bioorg. Med. Chem. Lett. 16(12) , 3268-72, (2006)
Full Text: HTML
Abstract
A series of substituted pyrrolidine-2,4-dicarboxylic acid amides were synthesized as potential antidiabetic agents, and many of them showed good in vitro DPP-IV inhibition (IC50 = 2-250 nM) with selectivity over DPP-II, DPP8, and FAP enzymes. Selected compounds 8c and 11a showed in vivo plasma DPP-IV inhibition after oral administration in Wistar rats.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
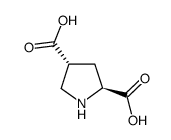 |
trans-4-Carboxy-L-proline
CAS:64769-66-0 |
C6H9NO4 |
Related Articles:
More...
|
Evidence for a role of GABA- and glutamate-gated chloride ch...
2012-11-01 [Pharmacol. Biochem. Behav. 103(1) , 69-75, (2012)] |
|
The effects of local perfusion of DAMGO on extracellular GAB...
2008-02-01 [J. Neurochem. 104(3) , 806-17, (2008)] |
|
Effects of theanine, r-glutamylethylamide, on neurotransmitt...
2005-08-01 [Nutr. Neurosci. 8(4) , 219-26, (2005)] |
|
Dissociation between hippocampal neuronal loss, astroglial a...
2006-12-01 [Neurochem. Int. 49(7) , 691-7, (2006)] |
|
Cerebral neurons of transgenic ALS mice are vulnerable to gl...
2006-06-01 [Exp. Neurol. 199(2) , 281-90, (2006)] |