Safety, tolerability and pharmacokinetics of trimebutine 3-thiocarbamoylbenzenesulfonate (GIC-1001) in a randomized phase I integrated design study: single and multiple ascending doses and effect of food in healthy volunteers.
Jean-Michel Paquette, Marianne Rufiange, Mirela Iovu Niculita, Julie Massicotte, Marc Lefebvre, Patrick Colin, Ariles Telmat, Maxime Ranger
Index: Clin. Ther. 36(11) , 1650-64, (2014)
Full Text: HTML
Abstract
Trimebutine 3-thiocarbamoylbenzenesulfonate (GIC-1001) is a new drug intended to be used for the management of visceral pain in patients undergoing sedation-free, full colonoscopy. The objectives of this Phase I, single-center, randomized, double-blinded, placebo-controlled, integrated study were to evaluate the safety and pharmacokinetics of GIC-1001 after single ascending doses (SAD) and multiple ascending doses (MAD) and to evaluate the influence of food on the pharmacokinetics in healthy volunteers.GIC-1001 or placebo was orally administered to 80 healthy male and female subjects (non- or ex-smokers) aged 18 to 50 years with a body mass index between 18.5 and 30 kg/m(2). The SAD portion of the study consisted of 5 cohorts with dose levels of 125 to 1000 mg. The MAD portion included 4 cohorts in which subjects received TID doses of 125 to 500 mg over 7 days (19 consecutive doses). Subjects were randomized (6:2) to receive GIC-1001 or placebo. The third portion of the study included a single 375-mg dose of GIC-1001 in a randomized, 2-period, crossover design to assess the influence of food (n = 8 subjects). Safety was evaluated by using adverse events (AEs), vital signs, ECGs, physical examination, cardiac monitoring, and laboratory test results. The analytes were assayed by using validated HPLC-MS/MS methods. Pharmacokinetic parameters were evaluated by using a noncompartmental analysis, and regression models were used to assess dose linearity. To evaluate the effect of food, 90% CIs of the ratio of geometric least squares means from ln-transformed pharmacokinetic parameters were calculated.The most frequently reported drug-related AEs were of nervous system and gastrointestinal origin. The most common AEs included headache, somnolence, and nausea. After single-dose administration, Tmax of trimebutine ranged from 1.0 to 1.5 hours. Cmax and AUCT were linear (nonlinearity P ≥ 0.05) and proportional (P < 0.05) over the studied dose range. Food increased the Cmax and AUC of trimebutine; the ratio of geometric least squares means (90% CI) were 140% (84-234) and 174% (138-221), respectively. In the MAD study portion, the Tmax of trimebutine ranged from 0.5 to 2 hours and AUCτ increased from 38 to 170 ng · h/mL. AUCτ and Cmax were linear and proportional over the studied dose range.GIC-1001 was well tolerated, and its safety profile was similar to that of placebo. Pharmacokinetics of GIC-1001 and its metabolites were mainly linear and proportional over the studied dose ranges. Steady state was generally considered to be reached after 3 days. Food consumption affected the pharmacokinetic profile of the analytes differently. (ClinicalTrials.gov identifier: NCT01738425.).Copyright © 2014 Elsevier HS Journals, Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
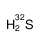 |
sulfur-32
CAS:13981-57-2 |
H2S | |
 |
Trimebutine
CAS:39133-31-8 |
C22H29NO5 |
|
LASP1 is a novel BCR-ABL substrate and a phosphorylation-dep...
2014-07-30 [Oncotarget 5(14) , 5257-71, (2014)] |
|
Use of sulfur-oxidizing bacteria as recognition elements in ...
2015-01-01 [Biotechnol. Appl. Biochem. 62 , 349-56, (2015)] |
|
Biogeochemical insights into microbe-mineral-fluid interacti...
2015-05-01 [Extremophiles 19(3) , 597-617, (2015)] |
|
Identification of SHIP-1 and SHIP-2 homologs in channel catf...
2015-07-01 [Dev. Comp. Immunol. 51 , 79-87, (2015)] |
|
Pedobacter bambusae sp. nov., isolated from soil of a bamboo...
2015-02-01 [Antonie van Leeuwenhoek 107(2) , 565-73, (2015)] |