Population pharmacokinetics of halofantrine in healthy volunteers and patients with symptomatic falciparum malaria.
Kerenaftali Klein, Leon Aarons, Feiko O Ter Kuile, Francois Nosten, Nick J White, Michael D Edstein, Paktiya Teja-Isavadharm
Index: J. Pharm. Pharmacol. 64(11) , 1603-13, (2012)
Full Text: HTML
Abstract
To investigate the population pharmacokinetics of the antimalarial halofantrine (HF) in healthy volunteers and patients with symptomatic falciparum malaria.Healthy volunteer data were obtained from six volunteers who received three different doses of HF (250, 500 and 1000 mg) after an overnight fast with a washout period of at least 6 weeks between doses. Patient data (n = 188) were obtained from randomised controlled trials conducted on the Thai-Burmese border in the early 1990s. They were either assigned to receive a total HF dose of 24 mg/kg (8 mg/kg every 6 h for 24 h) or 72 mg/kg (8 mg/kg every 6 to 10 h for 3 days). The population pharmacokinetics of HF were evaluated using non-linear mixed effects modelling with a two-compartment model with first-order absorption.The population estimates of apparent clearance (CL), volume of compartment one (V1), distributional clearance (CLD) and volume of compartment two (V2) of HF in healthy volunteers were 2453 l/day (102 l/h), 2386 l, 716 l/day (29.8 l/h) and 2641 l, respectively. The population estimates of the PK parameters in patients were 429 l/day (17.9 l/h), 729 l, 178 l/day (7.42 l/h) and 1351 l, respectively. All PK parameters were significantly related to body weight and some were related to sex, sampling method, pre-treatment parasite density and whether patients vomited or not. When the two datasets were analysed jointly using a maximum likelihood method, the population estimates in patients were 196 l/day (8.17 l/h), 161 l, 65 l/day (2.71 l/h) and 89 l, respectively, and the parameters were significantly related to body weight and sex. Bayesian analysis of the patient data, with a diffuse prior based on the healthy volunteer data analysis results, yielded the population estimates 354 l/day (14.8 l/h), 728 l, 162 l/day (6.75 l/h) and 1939 l, respectively.The pharmacokinetic properties of HF in patients with malaria are affected by several demographic variables as well as other relevant covariates. Apparent differences between the healthy volunteer and the patient data analysis results are not entirely due to differences in bioavailability. For the patient data analysis, the Bayesian method was preferred, as the fitting procedure was more stable, allowing random effects to be estimated for all four dispositional parameters.© 2012 The Authors. JPP © 2012 Royal Pharmaceutical Society.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
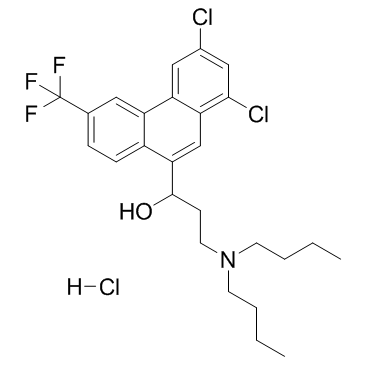 |
Halofantrine hydrochloride
CAS:36167-63-2 |
C26H31Cl3F3NO |
|
Effect of serum lipoproteins on stereoselective halofantrine...
2012-07-01 [Chirality 24(7) , 558-65, (2012)] |
|
Discordant temporal evolution of Pfcrt and Pfmdr1 genotypes ...
2012-03-01 [Antimicrob. Agents Chemother. 56(3) , 1382-9, (2012)] |
|
Using resin to generate a non-invasive intestinal bile-deple...
2012-09-29 [Eur. J. Pharm. Sci. 47(2) , 347-51, (2012)] |
|
CDA: combinatorial drug discovery using transcriptional resp...
2012-01-01 [PLoS ONE 7(8) , e42573, (2012)] |
|
Acute hypertriglyceridemia promotes intestinal lymphatic lip...
2011-08-01 [Mol. Pharm. 8(4) , 1132-9, (2011)] |