| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Atto 425
CAS:652966-03-5 |
|
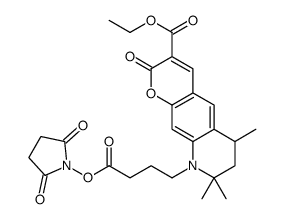 |
Atto 425-NHS ester
CAS:892156-28-4 |