| Structure | Name/CAS No. | Articles |
|---|---|---|
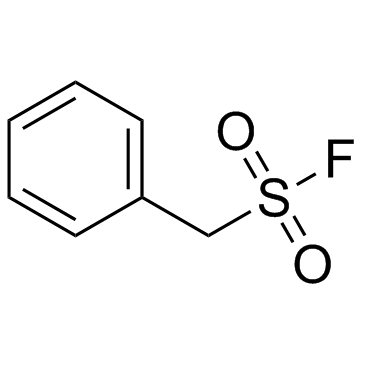 |
PMSF
CAS:329-98-6 |
|
 |
Suc-Ala-Ala-Pro-Phe-AMC
CAS:88467-45-2 |