| Structure | Name/CAS No. | Articles |
|---|---|---|
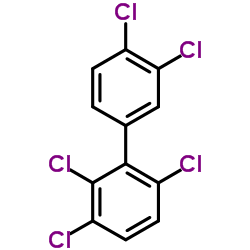 |
2,3,3',4',6-PCB
CAS:38380-03-9 |
M Li, C Rhine, L G Hansen
Index: Arch. Environ. Contam. Toxicol. 35(1) , 97-103, (1998)
Full Text: HTML
Polychlorinated biphenyls (PCBs) with the labile 2,3,6-substitution are important components of atmospheric and certain food chain exposures, but little is known about their biological activities. Chlorobiphenyl 110 (2,3,3',4',6-pentaCB) was investigated in weanling female rats dosed ip on days 21 and 22 and killed on day 23 of age. The initial preparation of CB 110 markedly induced 7-ethoxyresorufin O-dealkylase (EROD) activity and was found to be contaminated with coplanar 3,3',4,4',5-pentaCB (CB 126). The contaminated preparation (CB 110C) was purified with activated charcoal (CB 110P). The CB 110P induced pentoxyresorufin O-dealkylase (PROD), was weakly uterotropic and a modest depleter of serum thyroxine (T4). CB 110C caused increased liver weight, induced EROD, PROD, and UDP glucuronyl transferase activities and caused a greater depletion of serum T4; on the other hand, it suppressed the PROD induction and the uterotropic effect of CB 110P. Hepatic residues of CB 110 were a constant 2-3% of the dose while those of CB 126 (from CB 110C) increased with increasing dose to as much as 50% of the dose.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
2,3,3',4',6-PCB
CAS:38380-03-9 |
C12H5Cl5 |
|
Disposition and depuration of lindane (gamma-HCH) and polych...
2001-10-01 [Environ. Toxicol. Chem. 20(10) , 2377-82, (2001)] |
|
Persistence of 2,3,6-substituted pentachlorobiphenyls in the...
1981-01-01 [Toxicology 21(4) , 317-22, (1981)] |
|
Polychlorinated biphenyls (PCBs) and their congener-specific...
2002-08-01 [Food Addit. Contam. 19(8) , 779-95, (2002)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2024 ChemSrc All Rights Reserved