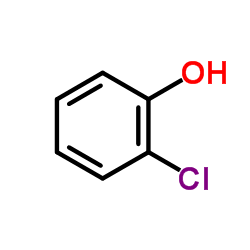
|
~% |
|
~89% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~% |
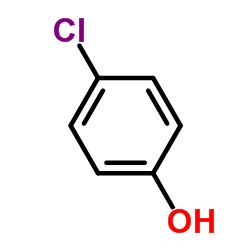
![[1,1'-Biphenyl]-2-ol,5-chloro Structure](https://image.chemsrc.com/caspic/313/607-12-5.png)
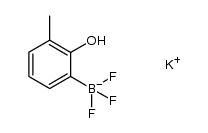
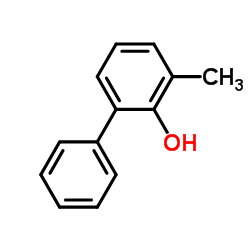
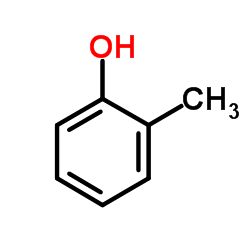

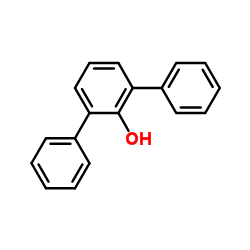

![4'-methyl[1,1'-biphenyl]-2-ol Structure](https://image.chemsrc.com/caspic/471/7374-34-7.png)
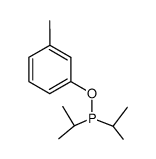
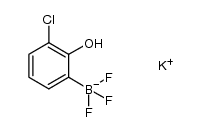
![[1,1'-Biphenyl]-2-ol,3-chloro Structure](https://image.chemsrc.com/caspic/419/85-97-2.png)