The treatment of hepatic encephalopathy.
Marsha Y Morgan, A Blei, K Grüngreiff, R Jalan, G Kircheis, G Marchesini, O Riggio, Karin Weissenborn
Index: Metab. Brain Dis. 22(3-4) , 389-405, (2007)
Full Text: HTML
Abstract
Current recommendations for the treatment of hepatic encephalopathy are based, to a large extent, on open or uncontrolled trials, undertaken in very small numbers of patients. In consequence, there is ongoing discussion as to whether the classical approach to the treatment of this condition, which aims at reducing ammonia production and absorption using either non-absorbable disaccharides and/or antibiotics, should be revisited, modified or even abandoned. Pros and cons of present therapeutic strategies and possible future developments were discussed at the fourth International Hannover Conference on Hepatic Encephalopathy held in Dresden in June 2006. The content of this discussion is summarized.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
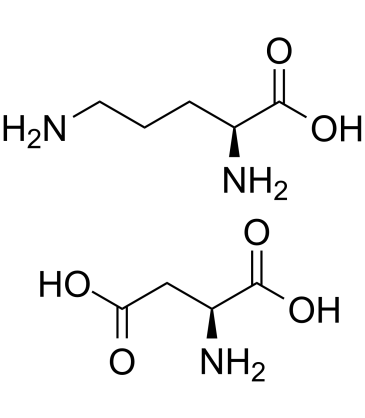 |
L-Ornithine L-aspartate salt
CAS:3230-94-2 |
C9H19N3O6 |
|
Modulation of neural activation following treatment of hepat...
2013-03-12 [Neurology 80(11) , 1041-7, (2013)] |
|
L-Ornithine-l-aspartate in the management of hepatic encepha...
2009-01-01 [J. Gastroenterol. Hepatol. 24(1) , 9-14, (2009)] |
|
A double-blind, randomized, placebo-controlled trial of intr...
2010-04-01 [Liver Int. 30(4) , 574-82, (2010)] |
|
Randomised clinical trial: L-ornithine-L-aspartate reduces s...
2014-07-01 [Aliment. Pharmacol. Ther. 40(1) , 63-71, (2014)] |
|
Hepatic encephalopathy following transjugular intrahepatic p...
2007-03-01 [Metab. Brain Dis. 22(1) , 45-50, (2007)] |