Biochemistry (Washington)
1998-07-07
High-efficiency incorporation in vivo of tyrosine analogues with altered hydroxyl acidity in place of the catalytic tyrosine-14 of Delta 5-3-ketosteroid isomerase of Comamonas (Pseudomonas) testosteroni: effects of the modifications on isomerase kinetics.
B Brooks, R S Phillips, W F Benisek
Index: Biochemistry 37(27) , 9738-42, (1998)
Full Text: HTML
Abstract
Versions of the Y55F/Y88F modified form of Delta 5-3-ketosteroid isomerase in which the active-site tyrosine-14 is replaced by 2-fluorotyrosine, 3-fluorotyrosine, and 2,3-difluorotyrosine, amino acids having progressively greater acidity of their phenolic hydroxyls, have been expressed in an Escherichia coli host and purified to high homogeneity. The steady-state kinetic properties of Y55F/Y88F KSI and its fluorotyrosine modified forms have been determined. The mechanistic implications of the results are presented and discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
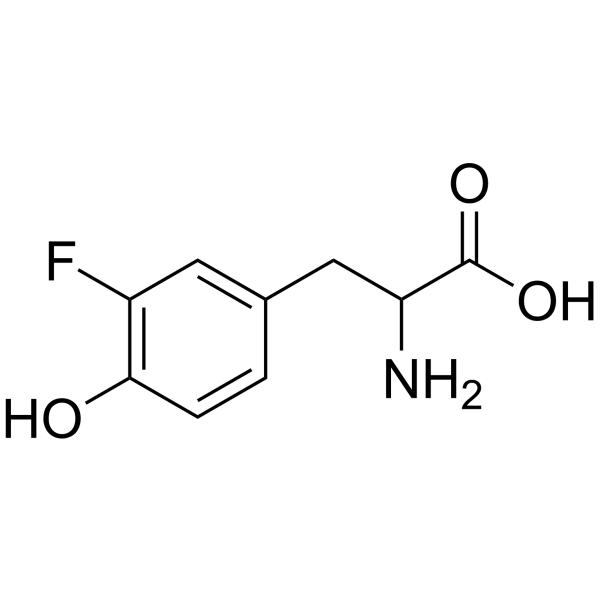 |
Tyrosine, 3-fluoro
CAS:403-90-7 |
C9H10FNO3 |
Related Articles:
More...
|
Aminoacrylate intermediates in the reaction of Citrobacter f...
2006-08-08 [Biochemistry 45(31) , 9575-83, (2006)] |
|
Hydrogen bonding in human manganese superoxide dismutase con...
2005-12-01 [Biophys. J. 89(6) , 4171-9, (2005)] |
|
Conformational changes in the crystal structure of rat gluta...
1998-08-14 [J. Mol. Biol. 281(2) , 323-39, (1998)] |
|
6-[18F]fluoro-L-m-tyrosine: metabolism, positron emission to...
1997-03-07 [Brain Res. 750(1-2) , 264-76, (1997)] |
|
Structural and spectral response of Aequorea victoria green ...
2005-03-15 [Biochemistry 44(10) , 3663-72, (2005)] |