| Structure | Name/CAS No. | Articles |
|---|---|---|
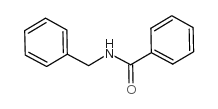 |
Benzamide, N-(phenylmethyl)-
CAS:1485-70-7 |
|
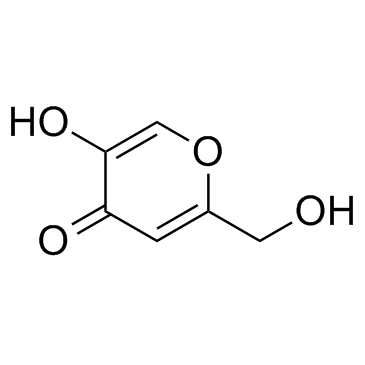 |
kojic acid
CAS:501-30-4 |