Kinetic determination of propranolol in tablets by oxidation with ceric sulphate.
S M Sultan, S A Altamrah, A M Aziz Alrahman, I Z Alzamil, M O Karrar
Index: J. Pharm. Biomed. Anal. 7(3) , 279-86, (1989)
Full Text: HTML
Abstract
A simple and accurate kinetic method for the determination of propranolol has been developed. Cerium(IV) sulphate (0.5 M) is used to oxidize propranolol in 2 M sulphuric acid at room temperature to the ketone form that absorbs light at a lambda max of 525 nm. The fixed-concentration method is used by recording the exact time, t(s), taken for the reaction to reach a fixed absorbance of 0.100. The unknown concentration, c(M), of propranolol is calculated from the equation: l/t = 0 0.000217 + 0.03 c. The method has been applied to the determination of propranolol in proprietary tablets and the results were compared with those obtained by the B.P. and other standard methods.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
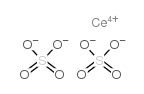 |
ceric sulfate
CAS:13590-82-4 |
CeO8S2 |
|
Differential in vitro inhibition studies of some cerium vana...
2015-04-01 [J. Enzyme Inhib. Med. Chem. 30(2) , 286-9, (2015)] |
|
Microsphaerol and seimatorone: two new compounds isolated fr...
2015-02-01 [Chem. Biodivers. 12(2) , 289-94, (2015)] |
|
Oxidation, deformation, and destruction of carbon nanotubes ...
2005-02-03 [J. Phys. Chem. B 109(4) , 1400-7, (2005)] |
|
Investigation of the NMR relaxation rate dose-response of a ...
2002-06-01 [Appl. Radiat. Isot. 56(6) , 895-9, (2002)] |
|
Chemiluminescence determination of ferulic acid by flow-inje...
2008-11-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 71(1) , 204-8, (2008)] |