Studies on electrochemical oxidation mechanisms of polyamides containing N-methylpyrrole and N-methylimidazole by electrospray ionization tandem mass spectrometry.
Zhonghua Ji, Jingjian Li, Jiang Zhou, Limei Hui, Gu Yuan, Shengmin Cai
Index: J. Phys. Chem. B 109(36) , 17162-8, (2005)
Full Text: HTML
Abstract
Electrochemical oxidization mechanism of polyamides containing N-methylpyrrole and N-methylimidazole was investigated by electrospray ionization tandem mass spectrometry (ESI-MS(n)). The spectra of the oxidization products unambiguously revealed that one or two oxygen atoms are added to the polyamide after the bulk electrolysis. It was found that electrochemical oxidation preferably takes place on the imidazole ring of the polyamide with both imidazole and pyrrole to produce a carbonyl group on the rings. ESI tandem mass spectrometry has proven to be an excellent method for the structural identification of electrochemical oxidation products of DNA-recognizing polyamides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
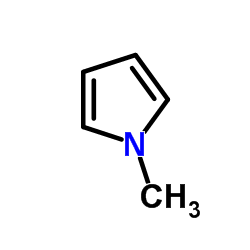 |
1-Methylpyrrole
CAS:96-54-8 |
C5H7N |
|
New insights on the photodissociation of N-methylpyrrole: th...
2009-12-31 [J. Phys. Chem. A 113(52) , 14554-8, (2009)] |
|
Investigation of non-covalent complexes of HIV-1 promoter DN...
2006-01-01 [Rapid Commun. Mass Spectrom. 20(11) , 1736-40, (2006)] |
|
Analysis of noncovalent complexes between human telomeric DN...
2005-02-04 [Chemistry 11(4) , 1157-62, (2005)] |
|
Investigation of noncovalent complexes between beta-cyclodex...
2006-01-01 [J. Am. Soc. Mass Spectrom. 17(1) , 9-14, (2006)] |
|
A new method for the determination of the relative affinity ...
2009-08-01 [J. Mass Spectrom. 44(8) , 1171-81, (2009)] |