Domain structural flexibility in rhodanese examined by quenching of a phosphorescent probe.
H Koloczek, J M Vanderkooi
Index: Biochim. Biophys. Acta 916(2) , 236-44, (1987)
Full Text: HTML
Abstract
Rhodanese (thiosulfate:cyanide sulfurtransferase, EC 2.8.1.1) is an enzyme composed of two domains with the catalytic site located in the bottom of the crevice formed by the two domains. In this work, rhodanese was labeled at its catalytic site with the phosphorescence probe eosin isothiocyanide. The accessibility of molecules to the probe was determined by phosphorescence lifetime-quenching studies. The phosphorescent probe was much more accessible to small molecules (I- and thiosulfate, radius about 3-5 A) than to a larger molecule (spin-label probe TEMPO, radius about 8-10 A). It was observed that a temperature-induced change in the rate of quenching occurred at around 28 degrees C. The results are interpreted in terms of structural fluctuations and displacement in the domains.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
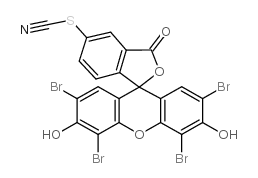 |
Eosin-5-isothiocyanate
CAS:60520-47-0 |
C21H7Br4NO5S |
|
Lys-430, site of irreversible inhibition of band 3 Cl- flux ...
1993-05-01 [Am. J. Physiol. 264(5 Pt 1) , C1155-64, (1993)] |
|
Avidin-EITC: an alternative to avidin-FITC in confocal scann...
1993-08-01 [J. Histochem. Cytochem. 41(8) , 1267-72, (1993)] |
|
The rotational diffusion of chloroplast phosphate translocat...
1989-06-01 [Eur. J. Biochem. 182(1) , 165-73, (1989)] |
|
Photohemolysis of human erythrocytes labeled in band 3 with ...
1986-10-01 [Photochem. Photobiol. 44(4) , 495-9, (1986)] |
|
Resonance energy transfer as a direct monitor of GTP-binding...
1991-07-23 [Biochemistry 30(29) , 7112-8, (1991)] |