| Structure | Name/CAS No. | Articles |
|---|---|---|
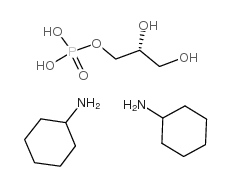 |
sn-Glycerol 3-phosphate biscyclohexylammonium salt
CAS:29849-82-9 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sn-Glycerol 3-phosphate biscyclohexylammonium salt
CAS:29849-82-9 |