| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
5-(Dimethylamino)-1-naphthalenesulfonohydrazide
CAS:33008-06-9 |
|
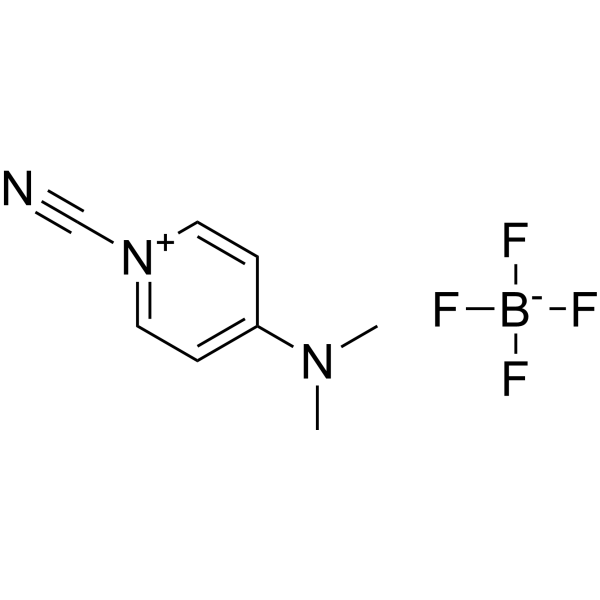 |
CDAP
CAS:59016-56-7 |