Thermochemistry and electronic structure of small boron and boron oxide clusters and their anions.
Minh Tho Nguyen, Myrna H Matus, Vu Thi Ngan, Daniel J Grant, David A Dixon
Index: J. Phys. Chem. A 113(17) , 4895-909, (2009)
Full Text: HTML
Abstract
Thermochemical properties of a set of small boron (B(n)) and boron oxide (B(n)O(m)) clusters, with n = 1-4 and m = 0-3, their anions, and the B(4)(2-) dianion, were calculated by using coupled-cluster theory CCSD(T) calculations with the aug-cc-pVnZ (n = D, T, Q, 5) basis sets extrapolated to the complete basis set limit with additional corrections. Enthalpies of formation, bond dissociation energies, singlet-triplet or doublet-quartet separation gaps, adiabatic electron affinities (EA), and both vertical electron attachment and detachment energies were evaluated. The predicted heats of formation show agreement close to the error bars of the literature results for boron oxides with the largest error for OBO. Our calculated adiabatic EAs are in good agreement with recent experiments: B (calc, 0.26 eV; exptl, 0.28 eV), B(2) (1.95, 1.80), B(3) (2.88, 2.820 +/- 0.020), B(4) (1.68, 1.60 +/- 0.10), BO (2.50, 2.51), BO(2) (4.48, 4.51), BOB (0.07), B(2)O(2) (0.37), B(3)O (2.05), B(3)O(2) (2.94, 2.94), B(4)O (2.58), and B(4)O(2) (3.14, 3.160 +/- 0.015). The BO bond is strong, so this moiety is maintained in most of the clusters. Thermochemical parameters of clusters are not linearly additive with respect to the number of B atoms. The EA tends to be larger in the dioxides. The growth mechanism of small boron oxides should be determined by a number of factors: (i) formation of BO bonds, (ii) when possible, formation of a cyclic B(3) or B(4), and (iii) combination of a boron cycle and a BO bond. When these factors compete, the strength of the BO bonds tends to compensate the destabilization arising from a loss of binding in the cyclic boron clusters, in such a way that a linear boron oxide prevails. When the B(2) moiety is present in these linear clusters, the oxide derivatives prefer a high spin state.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
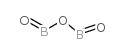 |
Boron oxide
CAS:1303-86-2 |
B2O3 |
|
Structure property correlation in lithium borophosphate glas...
2012-01-01 [Eur. Phys. J. E 35(1) , 8, (2012)] |
|
Structural investigations on sodium-lead borophosphate glass...
2012-04-12 [J. Phys. Chem. A 116(14) , 3547-55, (2012)] |
|
Spectroscopic investigations of Er3+ :CdO-Bi2 O3-B2O3 glasse...
2012-01-01 [Luminescence 27(5) , 334-40, (2012)] |
|
In vitro dissolution behavior of SiO2-MgO-Al2O 3-K2O-B2O3-F ...
2008-09-01 [J. Mater. Sci. Mater. Med. 19(9) , 3123-33, (2008)] |
|
Mixed alkali effect in Mn2+ doped 20ZnO+xLi2O+(30-x)K2O+50B2...
2013-01-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 101 , 140-7, (2013)] |