A single amino acid substitution in one of the lipases of Aspergillus nidulans confers resistance to the antimycotic drug undecanoic acid.
Ana G Brito-Madurro, Rolf A Prade, João M Madurro, Mário A Santos, Nalu T A Peres, Jeny R Cursino-Santos, Nilce M Martinez-Rossi, Antonio Rossi
Index: Biochem. Genet. 46(9-10) , 557-65, (2008)
Full Text: HTML
Abstract
A plausible approach to evaluate the inhibitory action of antifungals is through the investigation of the fungal resistance to these drugs. We describe here the molecular cloning and initial characterization of the A. nidulans lipA gene, where mutation (lipA1) conferred resistance to undecanoic acid, the most fungitoxic fatty acid in the C(7:0)-C(18:0) series. The lipA gene codes for a putative lipase with the sequence consensus GVSIS and WIFGGG as the catalytic signature. Comparison of the wild-type and LIP1 mutant strain nucleotide sequences showed a G --> A change in lipA1 allele, which results in a Glu(214) --> Lys substitution in LipA protein. This ionic charge change in a conserved LipA region, next to its catalytic site, may have altered the catalytic properties of this enzyme resulting in resistance to undecanoic acid.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
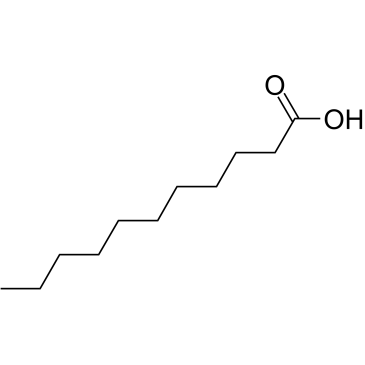 |
Undecanoic acid
CAS:112-37-8 |
C11H22O2 |
|
Use of multivariate statistical techniques to optimize the s...
2015-03-01 [Talanta 134 , 256-63, (2015)] |
|
Direct Derivatization vs Aqueous Extraction Methods of Fecal...
2015-07-01 [Lipids 50 , 681-9, (2015)] |
|
An efficient and economical MTT assay for determining the an...
2010-07-23 [J. Nat. Prod. 73 , 1193-5, (2010)] |
|
Chemical Modification of Graphene Oxide through Diazonium Ch...
2015-08-24 [Chemistry 21 , 12465-74, (2015)] |
|
Comparison of different protein immobilization methods on qu...
2001-12-15 [Anal. Biochem. 299(2) , 130-5, (2001)] |