Recovery of ammonium alum from waste solutions with a varying ratio of NH4 to Al in groundwater remediation after underground uranium leaching.
V Hostomská, J Hostomský
Index: J. Hazard. Mater. 147(1-2) , 342-9, (2007)
Full Text: HTML
Abstract
Experiments with cooling crystallization of ammonium alum, (NH(4)Al(SO(4))2.12H(2)O), were performed with concentrated multicomponent acidic solutions (originating from underground uranium leaching in Stráz pod Ralskem area, Czech Republic, and containing as the principal components Al3+, NH4+, and SO4(2-) ions) as well as with similar solutions prepared in the laboratory. The yield of NH(4)Al(SO(4))2.12H(2)O crystals increased significantly with the increasing NH4+/Al3+ molar ratio, in accordance with pertinent solubility data. The purifying effect of crystallization was quantified by means of the distribution coefficients, characterizing the uptake of ionic impurities to alum crystals; the tendency of cationic impurities to crystallize with NH(4)Al(SO(4))2.12H(2)O decreased in the following order: K+ >> Cr3+ >Na+ approximately Fe3+ >Mg2+ approximately Zn2+ >Fe2+. Additionally, gypsum (CaSO4.2H(2)O) solubilities at 25 degrees C, in mother liquors after NH(4)Al(SO(4))2.12H(2)O crystallization, were determined.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
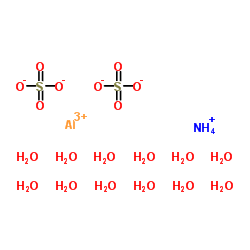 |
Ammonium aluminum disulfate dodecahydrate
CAS:7784-26-1 |
AlH31NO20S2 |
|
Aluminum ammonium sulfate dodecahydrate purified from tradit...
2012-06-01 [Biometals 25(3) , 541-51, (2012)] |
|
[A method for determining ammonium alum-based aerosol-formin...
1989-01-01 [Gig. Tr. Prof. Zabol. (2) , 46-7, (1989)] |
|
Removal of PCR inhibitors from soil DNA by chemical floccula...
2003-03-01 [J. Microbiol. Methods 52(3) , 389-93, (2003)] |
|
Evaluation of the reproductive and developmental toxicity of...
2011-09-01 [Food Chem. Toxicol. 49(9) , 1948-59, (2011)] |
|
A direct comparison of the taste of electrical and chemical ...
2008-06-01 [Chem. Senses 33(5) , 405-13, (2008)] |