Mechanism-based inactivation of rat liver cytochrome P-450 2B1 by 2-methoxy-5-nitrobenzyl bromide.
A P Armstrong, P F Hollenberg
Index: Drug Metab. Dispos. 27(6) , 741-5, (1999)
Full Text: HTML
Abstract
Mechanism-based inactivators serve as probes of enzyme mechanism, function, and structure. Koshland's Reagent II (2-methoxy-5-nitrobenzyl bromide, KR-II) is a potential mechanism-based inactivator of enzymes that perform O-dealkylations. The major phenobarbital-inducible form of cytochrome P-450 in male rat liver microsomes, CYP2B1, is capable of catalyzing O-dealkylations. The interactions of KR-II with purified CYP2B1 in the reconstituted system containing P-450, NADPH:P-450 oxidoreductase, and sonicated dilaurylphosphatidyl choline were studied. The benzphetamine N-demethylase activity of CYP2B1 was inactivated by KR-II in a time- and NADPH-dependent manner, and the loss of activity followed pseudo-first-order kinetics. The inactivation also required KR-II, and the rate of activity loss was dependent on the concentration of KR-II in a saturable fashion. The inactivator concentration required for the half-maximal rate of inactivation (KI) was approximately 0.1 mM. The inactivation was not prevented by the addition of the nucleophiles dithiothreitol and glutathione, nor was it reversed by gel filtration. The present results demonstrate that KR-II is a mechanism-based inactivator of rat CYP2B1.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
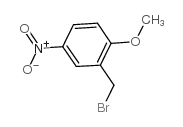 |
2-METHOXY-5-NITROBENZYL BROMIDE
CAS:3913-23-3 |
C8H8BrNO3 |
|
Chemical modification of mouse interferons.
1982-01-01 [J. Interferon Res. 2(2) , 177-85, (1982)] |
|
[Comparison of auc values for caffeine and its metabolites a...
2003-01-01 [Rocz. Panstw. Zakl. Hig. 54 Suppl , 56, (2003)] |
|
Effect of chemical modification on the cryoprecipitation of ...
1984-10-23 [Biochim. Biophys. Acta 790(2) , 125-31, (1984)] |
|
[Aldrichimica Acta 18 , 78, (1985)] |