Metabolism of N-methyl-amide by cytochrome P450s: formation and characterization of highly stable carbinol-amide intermediate.
Lionel Perrin, Nicolas Loiseau, François André, Marcel Delaforge
Index: FEBS J. 278(12) , 2167-78, (2011)
Full Text: HTML
Abstract
We report unambiguous proof of the stability of a carbinol intermediate in the case of P450 metabolism of an N-methylated natural cyclo-peptide, namely tentoxin. Under mild acidic or neutral conditions, the lifetime of carbinol-amide is long enough to be fully characterized. This metabolite has been characterized using specifically labeled (14) C-methyl tentoxin isotopomers, HPLC, HPLC-MS, MS-MS and NMR. Under stronger acidic conditions, the stability of this metabolite vanishes through deformylation. A theoretical mechanistic investigation reveals that the stability is governed by the accessibility of the nitrogen lone pair and its protonation state. For carbinol-amines, even in neutral conditions, the energy barrier for deformylation is low enough to allow rapid deformylation. Carbinol-amide behaves differently. Under neutral conditions, delocalization of the nitrogen lone pair increases the energy barrier of deformylation that is a slow process under such conditions. After protonation, we were able to optimize a deformylation transition that is lower in energy and thus accounts for the lower stability of carbinol-amides observed experimentally in acidic conditions. Finally, by considering the protocol usually used for extraction and analysis of this type of metabolite, carbinol-amide may thus be frequently ignored in drug metabolism pathways.© 2011 The Authors Journal compilation © 2011 FEBS.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
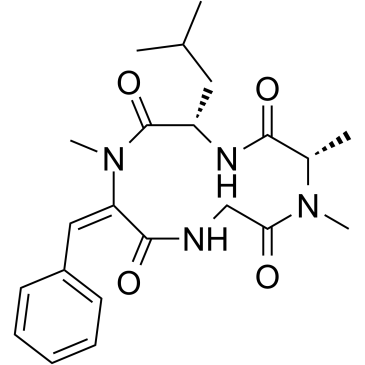 |
Tentoxin
CAS:28540-82-1 |
C22H30N4O4 |
|
[Simultaneous determination of four Alternaria toxins in app...
2010-12-01 [Se Pu 28(12) , 1128-31, (2010)] |
|
Hybrid Rhodospirillum rubrum F(0)F(1) ATP synthases containi...
2000-01-14 [J. Biol. Chem. 275(2) , 906-12, (2000)] |
|
Structure-activity relationships of cyclotetrapeptides: inte...
2001-01-01 [Adv. Exp. Med. Biol. 500 , 343-6, (2001)] |
|
Predicting the conformational states of cyclic tetrapeptides...
2003-07-01 [Biopolymers 69(3) , 363-85, (2003)] |
|
Interrelation between high and low affinity tentoxin binding...
1998-02-06 [J. Biol. Chem. 273(6) , 3343-50, (1998)] |