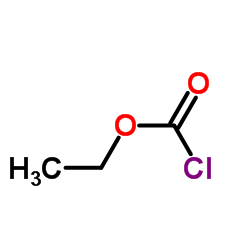
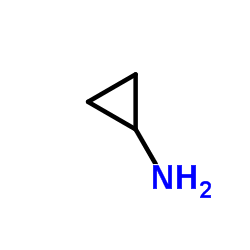
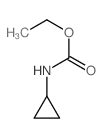
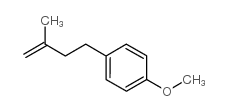
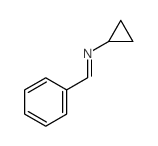
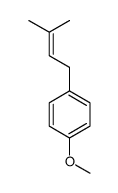
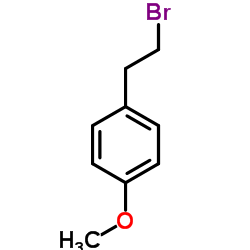
|
~97% |
|
~29% |
|
~99% |
|
~28% |
|
~% |