| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Isoniazid
CAS:54-85-3 |
|
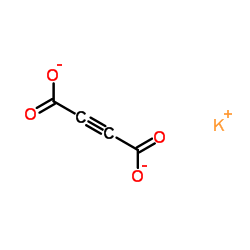 |
2-Butynedioate, potassium salt (1:1)
CAS:928-04-1 |
|
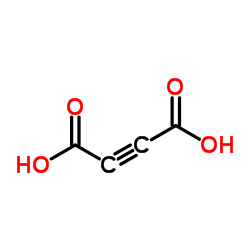 |
2-Butynedioic acid
CAS:142-45-0 |