| Structure | Name/CAS No. | Articles |
|---|---|---|
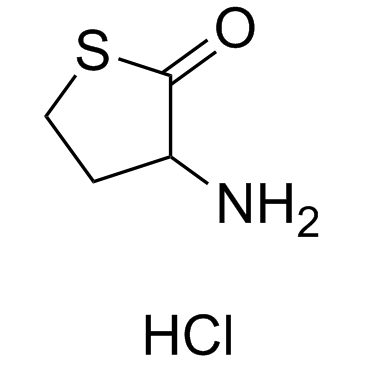 |
3-Aminodihydro-2(3H)-thiophenone hydrochloride
CAS:6038-19-3 |
|
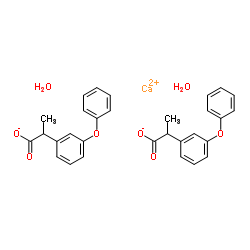 |
UNII:0X2CW1QABJ
CAS:53746-45-5 |