| Structure | Name/CAS No. | Articles |
|---|---|---|
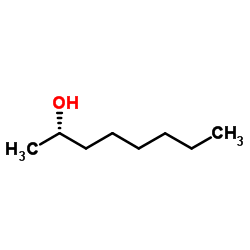 |
S-(+)-2-octanol
CAS:6169-06-8 |
|
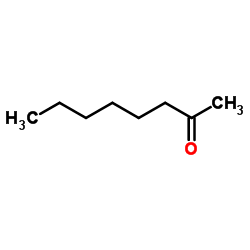 |
2-Octanone
CAS:111-13-7 |
|
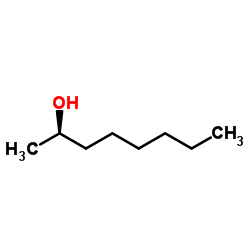 |
(R)-(−)-2-Octanol
CAS:5978-70-1 |
|
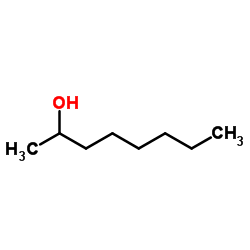 |
Octan-2-ol
CAS:123-96-6 |