Solid phase synthesis of anthraquinone peptides and their evaluation as topoisomerase I inhibitors.
Gregory I Giles, Ram P Sharma
Index: J. Pept. Sci. 11(7) , 417-23, (2005)
Full Text: HTML
Abstract
Anthraquinone peptide derivatives have previously been shown to inhibit the enzyme topoisomerase I (topo I), a pharmaceutical target for the prevention of malignant carcinomas. A highly efficient procedure for the attachment of the anthraquinone moiety to the N-terminus of a peptide on a solid support is reported. This methodology provides a convenient method for the synthesis of labelled peptides, with potential applications for chemotherapy, DNA detection and protein purification. As the synthetic strategy utilizes the solid phase, it should also be amenable to the generation of combinatorial libraries. The utility of the method by synthesizing a pool of peptides and assaying for topo I inhibition is demonstrated.Copyright (c) 2005 European Peptide Society and John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
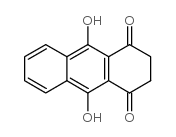 |
1,4-Anthracenedione,2,3-dihydro-9,10-dihydroxy
CAS:17648-03-2 |
C14H10O4 |
|
An investigation of anthraquinone dye biodegradation by immo...
2012-06-01 [Environ. Sci. Pollut. Res. Int. 19(5) , 1728-37, (2012)] |
|
An HPLC method development for the assessment of degradation...
2011-05-01 [Environ. Monit. Assess. 176(1-4) , 597-604, (2011)] |
|
Leucoquinizarin as an analytical spectrophotometric and fluo...
1986-04-01 [Analyst 111(4) , 429-33, (1986)] |
|
Reactions of 2, 3-dihydro-9, 10-dihydroxy-1, 4-anthracenedio...
[J. Org. Chem. 55(16) , 4960-61, (1990)] |
|
A comparison of vapour pressure measurements of quinizarin a...
[Color. Technol. 119(2) , 84-90, (2003)] |