19F nuclear magnetic resonance studies of selectively fluorinated derivatives of G- and F-actin.
M Brauer, B D Sykes
Index: Biochemistry 25(8) , 2187-91, (1986)
Full Text: HTML
Abstract
G-Actin is a globular protein (Mr 42 300) known to have three cysteine residues that are at least partially exposed and chemically reactive (Cys-10, -284, and -374). When G-actin was reacted with 3-bromo-1,1,1-trifluoropropanone, three resolvable 19F resonances were observed in the 19F NMR spectrum. This fluorinated G-actin derivative remained fully polymerizable, and its 31P NMR spectrum was not significantly different from that of unmodified G-actin, indicating that the chemical modification did not denature the actin and the modified residues do not interfere with the extent of polymerization or the binding of adenosine 5'-triphosphate. One of the three 19F resonances was assigned to fluorinated Cys-374 on the basis of its selective reaction with N-ethylmaleimide. This resonance was dramatically broadened after polymerization of fluorinated G-actin, while the other two resonances were not markedly broadened or shifted. Thus, Cys-10 and -284 are not involved in or appreciably affected by the polymerization of G-actin, while the mobility of the 19F label at Cys-374 is markedly reduced.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
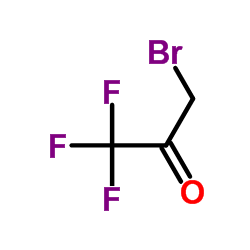 |
1-Bromo-3,3,3-trifluoroacetone
CAS:431-35-6 |
C3H2BrF3O |
|
Conformational change study of dengue virus NS2B-NS3 proteas...
2015-06-12 [Biochem. Biophys. Res. Commun. 461 , 677-80, (2015)] |
|
Actin dynamics studied by solid-state NMR spectroscopy.
1991-01-01 [Eur. Biophys. J. 19(3) , 147-55, (1991)] |
|
Substituted trifluoroketones as potent, selective inhibitors...
1987-06-15 [Biochem. Pharmacol. 36(12) , 1869-79, (1987)] |
|
Novel histone deacetylase inhibitors: cyclic tetrapeptide wi...
2004-11-01 [Bioorg. Med. Chem. Lett. 14(21) , 5343-6, (2004)] |
|
Interaction between G-actin and various types of liposomes: ...
1998-03-03 [Biochemistry 37(9) , 3149-55, (1998)] |