Reversed-phase ion-pair chromatography of tetracycline, tetracycline analogs, and their potential impurities.
J Hermansson, M Andersson
Index: J. Pharm. Sci. 71(2) , 222-9, (1982)
Full Text: HTML
Abstract
Methods are presented for the separation of tetracycline, tetracycline analogs, and their potential impurities by reversed-phase ion-pair chromatography. The mobile phase consisted of a phosphate buffer with tripropylamine or N,N-dimethyloctylamine as counterions and acetonitrile as the organic modifier. The chromatographic properties of the tetracyclines were significantly improved by addition of the tertiary amines. The best result was obtained with N,N-dimethyloctylamine as the counterion, which gave good separation efficiency and peak symmetry for most of the substances studies. Addition of the tertiary amines to the mobile phase also significantly affected the capacity factors of the tetracyclines. Stability studies of the tetracyclines showed a fast degradation of chlortetracycline to isochlortetracycline and of lymecycline to tetracycline in phosphate buffer solutions of different pH. The purity of pharmaceutical preparations of tetracyclines was also investigated.U
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
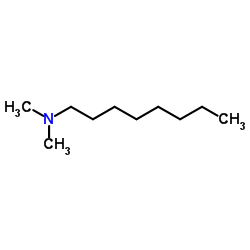 |
Octyldimethylamine
CAS:7378-99-6 |
C10H23N |
|
Determination of N,N-dimethyldodecylamine and N,N-dimethyloc...
2015-06-01 [Bull. Environ. Contam. Toxicol. 94 , 801-6, (2015)] |
|
Gaining insight in the behaviour of imidazolium-based ionic ...
2015-02-06 [J. Chromatogr. A. 1380 , 96-103, (2015)] |
|
Determination of (R)- and (S)-disopyramide in human plasma u...
[J. Chromatogr. A. 336(2) , 321-8, (1984)] |
|
A validated HPLC method for the determination of thiazinamiu...
2000-08-01 [J. Pharm. Biomed. Anal. 23(1) , 175-83, (2000)] |
|
The strongest isolable acid.
2004-10-11 [Angew. Chem. Int. Ed. Engl. 43(40) , 5352-5, (2004)] |