| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Caffeine
CAS:58-08-2 |
|
 |
Phosphorylase a, from rabbit muscle
CAS:9032-10-4 |
|
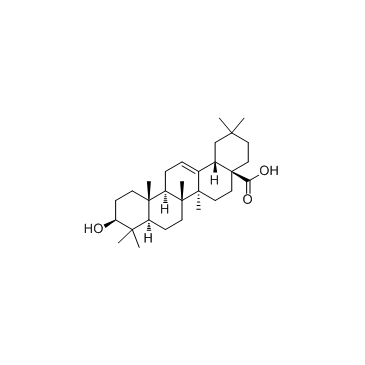 |
Oleanic acid
CAS:508-02-1 |