Lack of estrogenic potential of progesterone- or 19-nor-progesterone-derived progestins as opposed to testosterone or 19-nor-testosterone derivatives on endometrial Ishikawa cells.
J Botella, E Duranti, V Viader, I Duc, R Delansorne, J Paris
Index: J. Steroid Biochem. Mol. Biol. 55(1) , 77-84, (1995)
Full Text: HTML
Abstract
Estrogen receptors of human endometrial cancer Ishikawa cells were found to be present in moderate amounts (160-200 fmol/mg protein), and to specifically bind moxestrol (R2858) with a very high affinity characterized by a Kd around 60 pM, when measured under equilibrium conditions. The binding specificity respected a decreasing order as follows: estradiol (E2: 100%) > 4-hydroxy-tamoxifen (4OHTAM: 52.7%) > estriol (E3: 5.7%) > estrone (E1: 2.1%) > TAM (0.2%). The induction of alkaline phosphatase activity (APase) used as an estrogen-specific response, confirmed the intrinsic estrogenicity of progestins derived from 19-nor-testosterone (19NT): norethindrone (NOR), norethynodrel and levonorgestrel, at concentrations ranging from 10(-8) to 10(-6) M. The effect of NOR was partially blocked by the antiestrogen 4OHTAM, which was also partially agonistic in this model, but neither by the antiprogestin mifepristone (RU486) nor by the aromatase inhibitor aminoglutethimide. A simulatory effect was also detected at 10(-7) or 10(-6) M with ethindrone, the testosterone- (T) derived progestin homologous to NOR, and with both androgenic parent-compounds, i.e. T and 19NT themselves. In contrast, progesterone (P) derivatives like medroxyprogesterone acetate (MPA) and chlormadinone acetate (CMA) remained totally inactive, as well as 19-nor-progesterone (19NP) itself or its progestagenic derivatives: ORG 2058 and nomegestrol acetate (NOM). Structure-activity relationships deduced from these studies suggest that it is not the absence of the 19-methyl group which can account for the estrogenic potential of the so-called "19-norprogestins", but rather their steroid structure derived from T in a broad sense (including the 19NT derivatives), as opposed to the non-estrogenic therapeutic progestins derived from P like MPA or CMA, or from 19NP like NOM.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
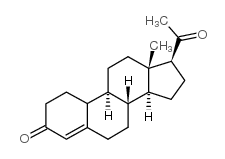 |
19-Norpregn-4-ene-3,20-dione
CAS:472-54-8 |
C20H28O2 |
|
Structure-activity relationships of synthetic progestins in ...
2008-05-01 [J. Steroid Biochem. Mol. Biol. 110(1-2) , 39-47, (2008)] |
|
The blood pressure and metabolic effects of 19-nor-deoxycort...
1984-01-01 [Clin. Exp. Hypertens. A. 6(9) , 1591-600, (1984)] |
|
The pharmacology of nomegestrol acetate.
2012-04-01 [Maturitas 71(4) , 345-53, (2012)] |
|
Interaction of new 19 nor progesterone derivatives with prog...
1986-01-01 [J. Pharmacol. 17(4) , 699-706, (1986)] |
|
19-Nor-corticosteroids in experimental and human hypertensio...
1982-01-01 [Clin. Exp. Hypertens. A. 4(9-10) , 1851-67, (1982)] |