Genotoxicity of stereoisomers of 1,2,3,4-diepoxybutane in the gpt gene of Chinese hamster ovary AS52 cells.
Min Young Kim
Index: Bull. Environ. Contam. Toxicol. 86(6) , 587-90, (2011)
Full Text: HTML
Abstract
Three optical isomers of 1,2,3,4-diepoxybutane, S,S-, R,R-, and meso-diepoxybutane, are produced by the metabolic processing of carcinogenic 1,3-butadiene. Our previous studies suggested that the observed differences between the biological effects of diepoxybutane optical isomers may be structural in their origin. Therefore, we examined the cytotoxicity and mutation fraction induced by three diepoxybutane stereoisomers in Chinese hamster ovary AS52 cells. All three stereoisomers reduced cell survival and increased gpt mutation fraction compared to the control; S,S-diepoxybutane exhibits the greatest cytotoxic and genotoxic potency, followed by R,R- and then meso-diepoxybutane. These results suggest that the cytotoxic and mutagenic effects of diepoxybutane are mediated by the stereochemical configurations of its isomers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
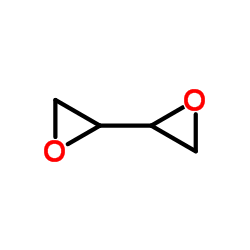 |
2,2′-bioxirane
CAS:1464-53-5 |
C4H6O2 |
|
Modelling Fanconi anemia pathogenesis and therapeutics using...
2014-01-01 [Nat. Commun. 5 , 4330, (2014)] |
|
DNA damage induced by three major metabolites of 1,3-butadie...
2012-09-18 [Mutat. Res. 747(2) , 240-5, (2012)] |
|
Alkyltransferase-mediated toxicity of bis-electrophiles in m...
2010-02-03 [Mutat. Res. 684(1-2) , 35-42, (2010)] |
|
Protective effect of acetyl-l-carnitine and α-lipoic acid ag...
2011-10-28 [Toxicology 289(1) , 52-8, (2011)] |
|
1,2:3,4-Diepoxybutane in blood of male B6C3F1 mice and male ...
2011-12-15 [Toxicol. Lett. 207(3) , 286-90, (2011)] |