Integration of reactive membrane extraction with lipase-hydrolysis dynamic kinetic resolution of naproxen 2,2,2-trifluoroethyl thioester in isooctane.
Chu-Hsun Lu, Yu-Chi Cheng, Shau-Wei Tsai
Index: Biotechnol. Bioeng. 79(2) , 200-10, (2002)
Full Text: HTML
Abstract
Lipases immobilized on polypropylene powders have been used as the biocatalyst in the enantioselective hydrolysis of (S)-naproxen from racemic naproxen thioesters in isooctane, in which trioctylamine was added to perform in situ racemization of the remaining (R)-thioester substrate. A detailed study of the kinetics for hydrolysis and racemization indicates that increasing the trioctylamine concentration can activate and stabilize the lipase as well as enhance the racemization and non-stereoselective hydrolysis of the thioester. Effects of the aqueous pH value and trioctylamine concentration on (S)-naproxen dissociation and partitioning in the aqueous phase as well as the transportation in a hollow fiber membrane were further investigated. Good agreements between the experimental data and theoretical results were obtained when the dynamic kinetic resolution process was integrated with a hollow fiber membrane to reactively extract the desired (S)-naproxen out of the reaction medium.Copyright 2002 Wiley Periodicals,
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
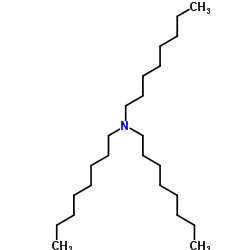 |
Trioctylamine
CAS:1116-76-3 |
C24H51N |
|
Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing a...
2015-11-24 [ACS Nano 9 , 11075-89, (2015)] |
|
Application of artificial neural network to investigate the ...
2015-01-01 [Sci. Rep. 5 , 16861, (2015)] |
|
Hollow fibre liquid phase micro-extraction by facilitated an...
2015-01-01 [Phytochem. Anal. 26 , 346-52, (2015)] |
|
Efficient removal of organic ligands from supported nanocrys...
2015-06-03 [J. Am. Chem. Soc. 137 , 6906-11, (2015)] |
|
[Analysis of the chemical constituents of essential oil from...
2010-03-01 [Zhong Yao Cai 33(3) , 385-7, (2010)] |