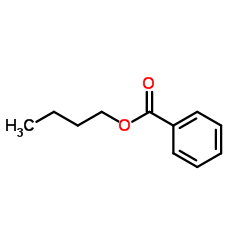
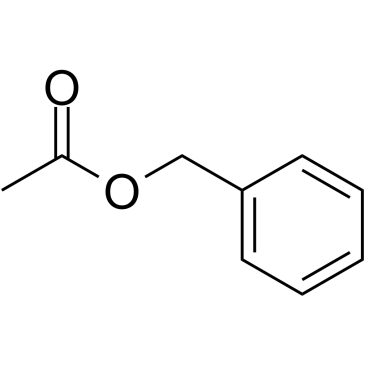
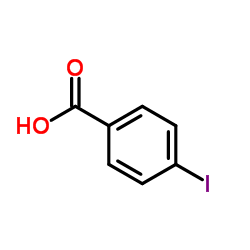
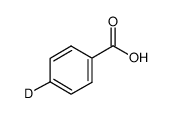
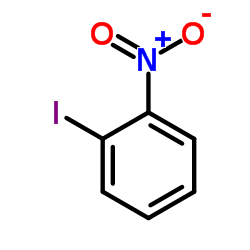
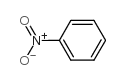
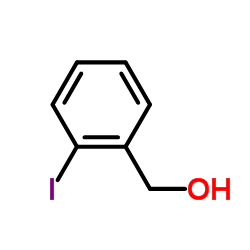
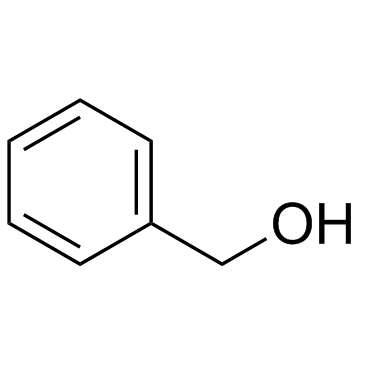
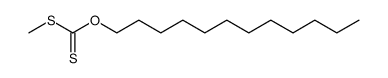
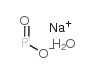
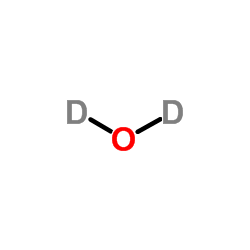
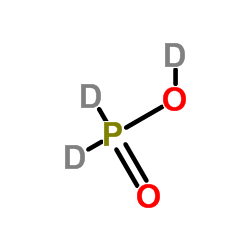
|
~96% |
|
~85% |
|
~97% |
|
~73% |
|
~99% |
|
~23% |
|
~97% |
|
~% |